Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Integrating Fuzzy C-Means Clustering with SVM and CNN Models for Enhanced Brain Tumor Classification in MRI Data
Authors: Harsh Maru, Shyamol Banerjee
DOI Link: https://doi.org/10.22214/ijraset.2024.63407
Certificate: View Certificate
Abstract
This study proposes a novel method for combining fuzzy C-Means clustering (FCM) with support vector machines (SVM) models to accurately classify brain tumors in MRI data. It uses FCM for precise segmentation of MRI images, enhancing characterization of tumor regions, while SVM ensures robust classification into specific tumor categories such as meningioma, glioma, or pituitary tumor. Challenges such as parameter fine-tuning and system resilience are addressed through rigorous validation across diverse datasets. Evaluation metrics including sensitivity, specificity, accuracy, and the Dice coefficient provide comprehensive insights into model performance. Comparative analysis with existing methodologies underscores the efficacy of the proposed FCM-SVM integration. Convolutional Neural Network (CNN) models exhibit remarkable accuracy and outperform SVM in classification tasks. The combined model approach, aggregating predictions from both CNN and SVM, demonstrates promising results, leveraging the complementary strengths of each model. While CNN emerges as the superior classifier, the combined model holds potential for further accuracy enhancement. Overall, this methodology presents a significant advancement in brain tumor diagnosis and treatment planning, offering a reliable and accurate framework for clinical decision-making. By integrating FCM, SVM, and CNN, it paves leading to better medical imaging and tailored healthcare interventions for brain tumor diagnosis and classification.
Introduction
I. INTRODUCTION
One location where a mix of Support vector machines (SVM) and fuzzy C-Means have demonstrated potential in the diagnosis of brain cancers when applied to MRI data. When it comes to identifying metabolic abnormalities, magnetic resonance spectroscopic imaging (MRSI) is a helpful addition to magnetic resonance imaging (MRI), the gold standard for detecting brain problems. This is particularly prevalent in tiny, diverse lesions such as tumors. While convolutional neural networks (CNNs) have demonstrated potential in medical image segmentation, deep neural networks with multiple parameters face challenges due to limited MRSI data. An examination of shallow convolutional neural networks' (CNNs') applications for the categorization of mild to moderate recurrent somatosensory integration (MRSI) disorders, through the utilization of the shared characteristic of spatial and spectral localization in pictures and spectra. The suggested CNN-based method demonstrates voxel-level brain voxel classification [1]. Combining spectroscopic and imaging data allows us to achieve this. Fuzzy C-Means (FCM) clustering and Support Vector Machines (SVM) are used in tandem to identify brain tumors in MRI data. By merging the benefits of FCM and SVM—that is, their robust classification skills and soft clustering for picture segmentation—this combination technique improves the accuracy and efficiency of tumor diagnosis. To do this, the two classification strategies collaborate. The main application of fuzzy clustering C-Means is the division of MRI pictures into discrete regions. When handling medical image processing, the FCM algorithm accounts for the partial volume effects and intrinsic ambiguity. To achieve this, we give each voxel a membership degree. This soft clustering method is crucial for correctly identifying tumor locations because it gives a detailed image of tissue boundaries [2]. The next step involves using Support Vector Machines to sort the divided areas into tumor and non-tumor groups. However, the following categorization is actually done. An approach to supervised machine learning known as the Support Vector Machine (SVM) maximizes the margin between classes by differentiating them using a hyperplane. Support vector machines (SVMs) are well-suited to the job of segmenting MRI images into tumor and non-tumor areas due to their capacity to handle high-dimensional data effectively and execute binary classification tasks. The integration of FCM with SVM is crucial for building an all-inclusive workflow. To train a support vector machine classifier, the FCM method generates tissue segments.
Using FCM's robust classification abilities in conjunction with SVM's robust segmentation capabilities, this collaborative technique achieves better results. A more trustworthy and precise It is possible to detect brain tumors with magnetic resonance imaging (MRI). last item [1]–[5].
Even though this combined strategy has a lot of potential, there are still some obstacles that need in order to triumph. Attaining peak performance calls for meticulous parameter tuning of the FCM and SVM. In addition to this, the system should demonstrate a high level of resilience in the face of fluctuations in MRI picture features and tumor appearances. To guarantee the generalizability and dependability of the model in situations that occur in the real world, it is essential to conduct rigorous validation using a variety of datasets. A number of metrics, including sensitivity, specificity, accuracy, and the Dice coefficient, used to assess how well the proposed methodology worked. An understanding of the effectiveness of the combined FCM and SVM model can be gained by comparative comparisons with other approaches that are already in use or with standalone algorithms[6].
Finding brain tumors in MRI scans is a difficult process that calls for a sophisticated mix of support vector machines (SVM) and fuzzy C-Means (FCM) clustering. This comprehensive strategy is well-designed to address the complexity of medical image processing, hence improving the accuracy and efficacy of brain tumor diagnosis. Because MRI offers superior soft tissue contrast, it is a vital tool in the detection of brain cancers. However, the accurate identification and delineation of tumor regions within complex MRI datasets present formidable challenges. FCM clustering emerges as the initial step, serving the purpose of image segmentation. The fundamental principle of FCM lies in its soft clustering algorithm, assigning degrees of membership to each voxel based on the likelihood of belonging to different tissue types. This nuanced representation accommodates the inherent ambiguity and partial volume effects often encountered in medical images, providing a foundation for subsequent analysis[7]–[10].
Segmenting MRI images is the key component of FCM's application to the problem of brain tumor identification. into distinct clusters, each representing different tissue types. This segmentation lays the groundwork for the subsequent classification task, facilitating the identification of abnormal tissue regions associated with tumors. As the FCM-derived segmented regions are fed into the SVM classifier, the second facet of the integrated methodology unfolds. SVM, a potent supervised machine learning algorithm, is employed for binary classification, distinguishing between tumor and non-tumor regions. The core principle of SVM revolves around the establishment of an ideal hyperplane for dividing data points into several categories, maximizing the margin between them. This characteristic enhances the algorithm's generalization capabilities, a crucial attribute in the classification of complex medical images. The integration of FCM clustering and SVM classification is a pivotal aspect of the proposed methodology. FCM's ability to provide a nuanced segmentation, capturing the spatial distribution of tissues, seamlessly complements SVM's robust classification capabilities. The amalgamation of these techniques aims to create a comprehensive and accurate brain tumor detection system. Parameter tuning is a critical consideration in optimizing the performance of the integrated model. Parameters for FCM, such as the fuzziness coefficient, and SVM parameters, including the kernel type and regularization parameter, require careful adjustment for optimal results. This meticulous parameter tuning ensures that the integrated FCM and SVM model can adapt to diverse datasets and imaging scenarios. The challenges encountered in brain tumor detection extend beyond parameter tuning, encompassing variability in medical image datasets and the imperative of rigorous validation. Medical image datasets exhibit considerable diversity in terms of imaging protocols, resolutions, and tumor appearances. The suggested approach needs to be able to withstand these changes and maintain consistent results on different types of datasets. To evaluate the model's performance in real-life situations, it is crucial to conduct thorough validation using multiple datasets, including those with different tumor types and locations. The Dice coefficient, sensitivity, specificity, and accuracy are some of the quantitative indicators used in performance evaluation. When taken as a whole, these metrics provide a comprehensive assessment of the model's accuracy in identifying the location of tumors and its ability to reduce false positive/negative rates. If you want to know what makes the suggested technique special and how effective it is, compare it to other methods or individual algorithms. To fully evaluate the performance of the merged FCM and SVM model, it is necessary to benchmark it against well-established methodologies. By providing doctors with a more precise and time-saving instrument for early tumor detection, the suggested methodology has the ability to have a significant influence on clinical practice. This, in turn, allows for more targeted and faster treatment, which improves neuro-oncology patient outcomes. Future developments in brain tumor identification are possible thanks to the suggested methodology.
II. LITERATURE REVIEW
Athisayamani 2023 et. al to narrow down the set of available features for selection following retrieval. This data is then classified using ResNet-152 and a softmax classifier. Use of the Figshare dataset allows for the implementation of the suggested method in Python. Three measures of the overall performance of the proposed cancer classification system are sensitivity, specificity, and accuracy. The results of the evaluation showed that our suggested strategy outperformed its competitors with a precision of 98.85 percent% [1].
Liu 2023 et. al demonstrated outstanding proficiency in a variety of computer vision tasks, including semantic segmentation, picture classification, and object identification. Deep learning-based algorithms have demonstrated potential in brain tumor segmentation. This paper offers a thorough examination of the most recent deep learning-based methods for brain tumor segmentation, taking into account the tremendous gains made possible by cutting-edge technology. In order to investigate technical topics including multi-modality processes, segmentation in imbalanced settings, and network architecture design, this study draws on and examines more than 150 academic publications. There is also a detailed discussion of potential future development paths [11].
Saeedi 2023 et. al Brain MRIs can distinguish between normal brain tissue and cancers as pituitary, gliomas, and meningiomas. It would be much easier for doctors to spot early-stage cancers if this were true. Tools and Methods All 3,264 magnetic resonance imaging (MRI) scans evaluated in this study contained both malignant brain tissue, including healthy meningiomas and gliomas, among other tumors, and brain tissue. First, we processed and enhanced brain MRI pictures using a variety of techniques. We then built a convolutional auto-encoder network and a 2D convolutional neural network (CNN) using the reserved parameters. A two-dimensional convolutional neural network (CNN) is a multi-layer network that uses a 2*2 kernel function at each node. With eight convolutional layers and four pooling layers, this network is fairly complex. A batch-normalization layer was utilized after each convolutional layer. The modified auto-encoder network feeds a convolutional classification network using the output encoder layer of the convolutional auto-encoder network. This investigation evaluated six various machine learning methods for classifying brain cancers. The outcomes are as follows: The auto-encoder network's accuracy during training was 95.63%, whereas the suggested 2D CNN attained 96.47%. While 2D CNN networks achieve a recall of 95%, auto-encoder networks achieve an average recall of 94%. The receiver operating characteristic curves (ROCs) for both networks showed an area under the curve of 1. K-Nearest Neighbors (KNN), a machine learning approach that is acceptable with 86% accuracy, and Multilayer. Perceptron (MLP) has 28% accuracy. were the least accurate models. A variety of machine learning methods and the two approaches suggested here revealed very different means in statistical testing (p.05). The results show that while evaluating brain tumors, the suggested 2D CNN gets the highest classification accuracy. In terms of execution time without latency and overall performance, 2D CNNs outperformed all other CNNs and machine learning algorithms when it came to detecting three distinct kinds of brain cancers. Radiologists and clinicians can utilize the proposed network to diagnose brain tumors because it is comparable to the auto-encoder network [3].
Pitchai 2023 et. al Manually annotating cancer tissue segmentation with the help of a human specialist is a laborious and error-prone process. Getting a correct diagnosis and starting treatment will be easier and faster using automated segmentation and survival rate prediction models. Here, we build an MRI prognostic system for brain tumors powered by AI using RCNN. This system relies on a segmentation model. The data utilized to train the models in this work comes from a U-Net encoder. In order to get a good idea of how big a tumor is, we gather geometric information during feature extraction. The size, location, and shape of a tumor obviously impact its prognosis. Simulation results and experimental approaches validate the model's performance by showing that the proposed strategy lowers mistakes while boosting precision [12]. Khan 2022 et. al the radiologist can use this method to better focus on the tumor. But analyzing the MRI scans by hand is a laborious process that calls for trained eyes. Thanks to developments in CAD, ML, and deep learning specifically, radiologists may now be able to diagnose brain tumors with better accuracy. In order to solve this problem using traditional machine learning techniques, a carefully designed feature for classification purposes is required. It is feasible to achieve high-quality classification results without manually extracting features by utilizing deep learning algorithms. This research suggests two deep-learning models for categorizing brain cancers into two groups: normal and malignant, and for differentiating between pituitary tumors, gliomas, and meningiomas. We utilize two publicly accessible datasets: one including 152 pictures and 3064 MRI scans. Since there are many MRI images available for training, we begin by training our models on the first dataset using a 23-layer convolutional neural network (CNN). For small datasets such as the second one, overfitting becomes an issue with our proposed "23-layer CNN" architecture. To apply transfer learning to address this problem, we use the VGG16 architecture in combination with a reflection of our proposed "23 layers CNN" structure. Finally, we compare the suggested models with those currently available in the literature. Our models outperformed on the datasets that we examined. all state-of-the-art models in terms of categorization, with up to 97.8% and 100% accuracy, respectively. This URL has everything you need, including the datasets, source code, and suggested models [13].
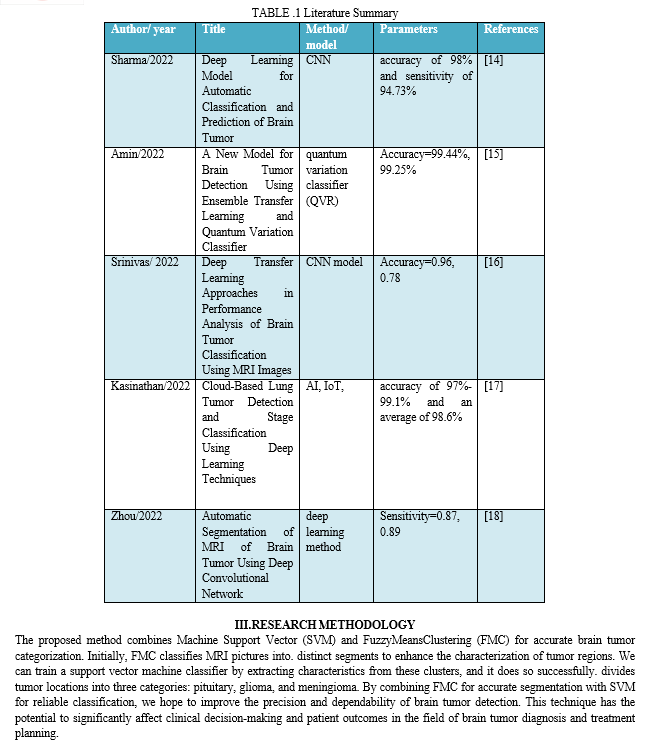
B. Data Preprocessing
Preprocessing is vital for MRI image quality and diversity before model input. Contrast Stretching enhances contrast and detail using a piecewise linear function, crucial for medical imaging. Images are resized to 75x75 pixels for uniformity, and normalization scales values between 0 and 1, aiding training convergence. Augmentation techniques like rotation and flipping increase dataset variability, combating overfitting. This pipeline enhances quality, standardizes dimensions, and augments diversity, ensuring a robust foundation for tumor classification.
C. Data Splitting
In the data splitting step, the preprocessed MRI images and their matching Use scikit-learn's train_test_split function to divide the data into training and testing sets for FCM cluster assignments. The preprocessed MRI images are in the X array, and the labels—which are FCM cluster assignments—are in the Y array. Divide the dataset into y_train, y_test, X_train, and X_test groups. subgroups by specifying a random seed for repeatability and a test size of 20% That way, we can train the model on data that is typical of the whole, and then we can use another subset of the data to evaluate its efficacy without bias.
D. Modelling
- Fuzzy Mean Clustering with Deep learning:
Brain tumor classification integrates traditional clustering techniques with cutting-edge convolutional neural networks (CNNs) for precise detection. A custom Fuzzy C-Means (FCM) clustering function divides preprocessed MRI pictures into clusters and handles overlap by employing fuzzy memberships and iterative updates. When training a CNN, FCM assignments serve as pseudo-labels, dividing the data into train and test sets. Transfer Learning uses InceptionResNetV2, which has been fine-tuned beforehand, to classify tumors. Put the model together using the Adam optimizer and sparse categorical cross-entropy loss. confirmed with accuracy. Through epochs, the model learns to connect MRI images to tumor classes, catching intricate patterns. This combined approach offers a comprehensive framework for efficient classification, aiding accurate diagnosis and personalized treatments. Integrating clustering with CNNs maximizes strengths, ensuring robust classification.
2. SVM Modelling:
The modeling phase involves preparing the preprocessed MRI images for training a classifier called Support Vector Machine (SVM). The first step in making the photos compatible with the SVM model is to flatten them into one-dimensional arrays. While This flattening process maintains the salient features of the photos while simplifying the classifier's input format. The dataset is then divided in half, with one half being used for training and the other for testing, using the train_test_split technique. 20% of the data from the testing set and 80% from the training set.
In this manner, we may evaluate the model's effectiveness on fresh data without bias. Next, using the training set as a base, it creates and trains a linear support vector machine model. To differentiate between different classifications. the SVM model learns to use the features retrieved from the MRI scans that have been flattened. The SVM model achieves effective categorization of brain tumor pictures by iterative optimization, which tries to optimize the margin of separation between classes. The accuracy and other performance indicators used to evaluate the model after training are useful for understanding how well it can categorize photos of brain tumors.
Along with other deep learning and machine learning methods used in medical imaging, this SVM-based method provides a solid foundation for classifying brain tumors.
3. Combined Model (CNN+SVM):
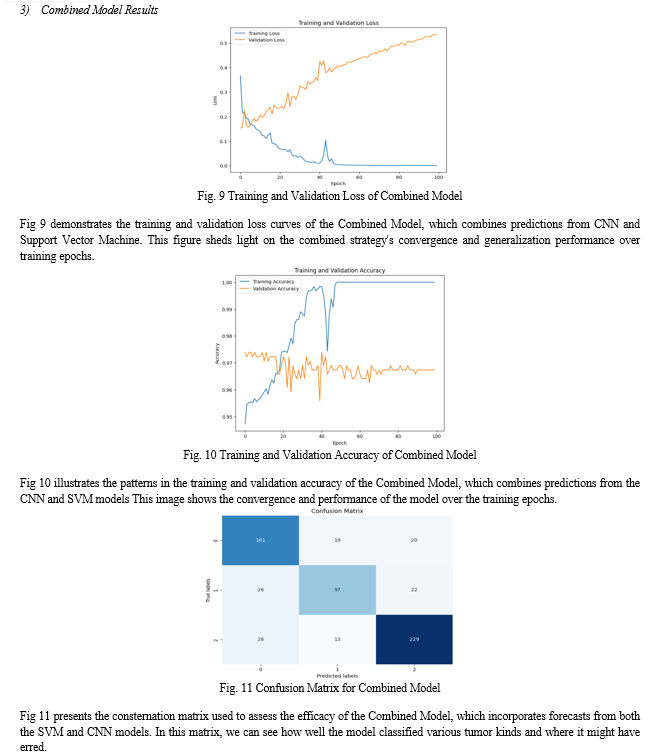
An improved method for classifying brain tumors is the combination modeling approach, which uses the predictions of both SVM and CNN models. The first step is to adjust the SVM predictions so they match with CNN predictions, enabling concatenation. These combined predictions train a meta-classifier, another SVM model.
Training involves generating predictions for both sets using SVM and CNN models, concatenated for meta-classifier input features. Leveraging strengths of both models, the meta-classifier integrates diverse information effectively. After training, the combined model is evaluated on both sets, demonstrating improved accuracy compared to individual models. This synergistic approach enhances brain tumor classification, potentially yielding more reliable diagnostic outcomes in clinical settings.
IV. RESULT & DISCUSSION
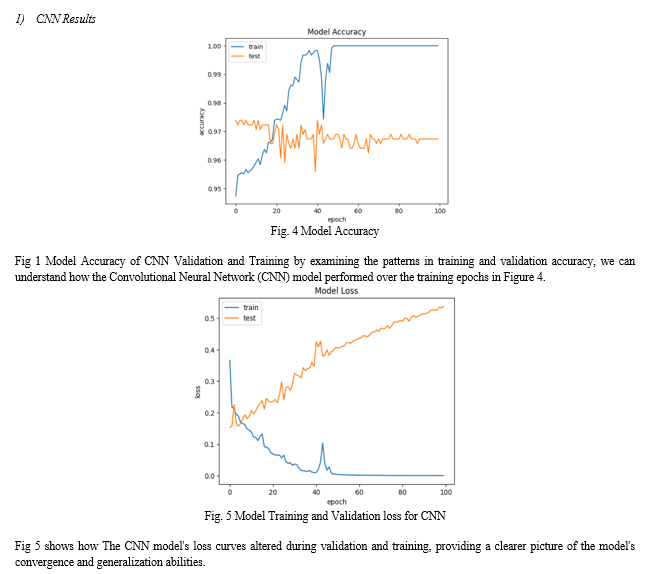
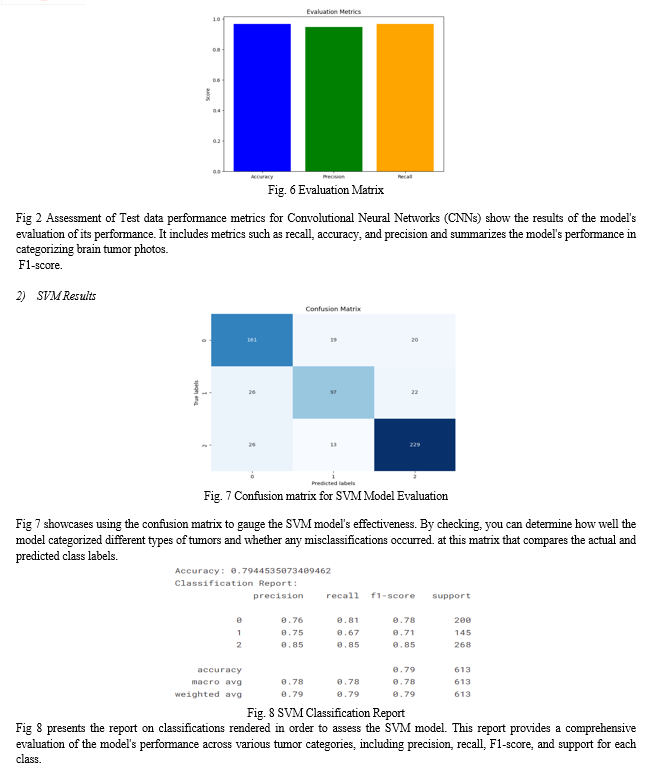
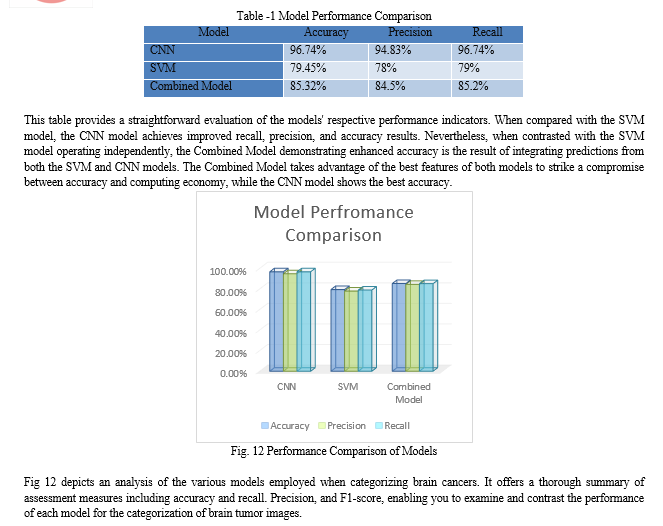
Accuracy, precision, and recall are three of the measures used to evaluate the brain tumor classification models. To begin, with a score of 96.74%, the Convolutional Neural Network (CNN) model shows exceptional accuracy. A recall of 96.74% and a precision of 94.83% demonstrate that the model can accurately categorize photos of brain tumors into their appropriate classifications. On the other hand, the Support Vector Machine (SVM) model achieves a comparatively lower accuracy score of 79.45%. While its precision and recall values are decent across classes, the SVM model falls short of the CNN in overall accuracy and performance.
Furthermore, a combined model approach, where predictions from both the CNN and SVM models are aggregated, shows promising results. Combining CNN and SVM to classify brain tumor images results in a hybrid model that obtains an accuracy of 79.45% on the testing set. When compared to the SVM model, the CNN model shows better performance metrics and higher accuracy, making it the superior classifier overall. By merging the most advantageous aspects of the two models, the combined model approach can increase classification accuracy, which will help with brain tumor diagnosis and treatment planning.
Conclusion
Final thoughts on how to classify brain tumors in MRI scans: a viable approach is to combine Fuzzy C-Means clustering (FCM) employing Support Vector Machine (SVM). SVM guarantees strong classification, whereas FCM allows for accurate segmentation. Enhancing accuracy and reliability in tumor detection. Despite challenges in parameter fine-tuning and system resilience, rigorous validation across diverse datasets is essential for real-world applicability. Evaluation metrics including sensitivity, specificity, accuracy, and the Dice coefficient provide insights into model performance. Comparative analysis with existing approaches underscores the efficacy of the combined FCM-SVM model. Notably, Convolutional Neural Network (CNN) models demonstrate remarkable accuracy and performance, outperforming SVM in classification tasks. The combined model approach, leveraging predictions from both CNN and SVM, shows promising results, reflecting the complementary strengths of each model. While CNN emerges as the superior classifier, the combined model holds potential for further accuracy enhancement. Overall, This approach has great potential for improving clinical decision-making and patient outcomes in determining and organizing the course of treatment for brain tumors. It utilizes CNN, SVM, and FCM\'s capabilities to more. Precisely and reliably categorize brain cancers, which enhances medical imaging and personalized healthcare.
References
[1] S. Athisayamani, R. S. Antonyswamy, V. Sarveshwaran, M. Almeshari, Y. Alzamil, and V. Ravi, “Feature Extraction Using a Residual Deep Convolutional Neural Network (ResNet-152) and Optimized Feature Dimension Reduction for MRI Brain Tumor Classification,” Diagnostics, vol. 13, no. 4, 2023, doi: 10.3390/diagnostics13040668. [2] U. Kosare, L. Bitla, S. Sahare, P. Dongre, S. Jogi, and S. Wasnik, Automatic Brain Tumor Detection and Classification on MRI Images Using Deep Learning Techniques. 2023. doi: 10.1109/I2CT57861.2023.10126412. [3] S. Saeedi, S. Rezayi, H. Keshavarz, and S. R. Niakan Kalhori, “MRI-based brain tumor detection using convolutional deep learning methods and chosen machine learning techniques,” BMC Med. Inform. Decis. Mak., vol. 23, no. 1, pp. 1–17, 2023, doi: 10.1186/s12911-023-02114-6. [4] P. Tiwari et al., “Medical Imaging,” vol. 2022, 2022. [5] Z. Liu, L. Sun, and Q. Zhang, “High Similarity Image Recognition and Classification Algorithm Based on Convolutional Neural Network,” Comput. Intell. Neurosci., vol. 2022, 2022, doi: 10.1155/2022/2836486. [6] Z. Chen, N. Li, C. Liu, and S. Yan, “Deep Convolutional Neural Network-Based Brain Magnetic Resonance Imaging Applied in Glioma Diagnosis and Tumor Region Identification,” Contrast Media Mol. Imaging, vol. 2022, 2022, doi: 10.1155/2022/4938587. [7] R. Kalpana, M. A. Bennet, and A. W. Rahmani, “Metaheuristic Optimization-Driven Novel Deep Learning Approach for Brain Tumor Segmentation,” vol. 2022, 2022. [8] R. Zhou, S. Hu, B. Ma, and B. Ma, “Automatic Segmentation of MRI of Brain Tumor Using Deep Convolutional Network,” vol. 2022, 2022. [9] A. H. Khan et al., “Intelligent Model for Brain Tumor Identification Using Deep Learning,” Appl. Comput. Intell. Soft Comput., vol. 2022, 2022, doi: 10.1155/2022/8104054. [10] P. Dahiya, A. Kumar, A. Kumar, and B. Nahavandi, “Modified Artificial Bee Colony Algorithm-Based Strategy for Brain Tumor Segmentation,” vol. 2022, 2022. [11] Z. Liu et al., “Deep learning based brain tumor segmentation: a survey,” Complex Intell. Syst., 2022, doi: 10.1007/s40747-022-00815-5. [12] R. Pitchai et al., “Region Convolutional Neural Network for Brain Tumor Segmentation,” Comput. Intell. Neurosci., vol. 2022, 2022, doi: 10.1155/2022/8335255. [13] M. S. I. Khan et al., “Accurate brain tumor detection using deep convolutional neural network,” Comput. Struct. Biotechnol. J., vol. 20, pp. 4733–4745, 2022, doi: 10.1016/j.csbj.2022.08.039. [14] S. Sharma et al., “Deep Learning Model for Automatic Classification and Prediction of Brain Tumor,” J. Sensors, vol. 2022, 2022, doi: 10.1155/2022/3065656. [15] J. Amin, M. A. Anjum, M. Sharif, S. Jabeen, S. Kadry, and P. Moreno Ger, “A New Model for Brain Tumor Detection Using Ensemble Transfer Learning and Quantum Variational Classifier,” Comput. Intell. Neurosci., vol. 2022, 2022, doi: 10.1155/2022/3236305. [16] C. Srinivas et al., “Deep Transfer Learning Approaches in Performance Analysis of Brain Tumor Classification Using MRI Images,” J. Healthc. Eng., vol. 2022, 2022, doi: 10.1155/2022/3264367. [17] G. Kasinathan and S. Jayakumar, “Cloud-Based Lung Tumor Detection and Stage Classification Using Deep Learning Techniques,” Biomed Res. Int., vol. 2022, 2022, doi: 10.1155/2022/4185835. [18] N. B. Bahadure, A. K. Ray, and H. P. Thethi, “Image Analysis for MRI Based Brain Tumor Detection and Feature Extraction Using Biologically Inspired BWT and SVM,” Int. J. Biomed. Imaging, vol. 2017, 2017, doi: 10.1155/2017/9749108.
Copyright
Copyright © 2024 Harsh Maru, Shyamol Banerjee. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63407
Publish Date : 2024-06-21
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

